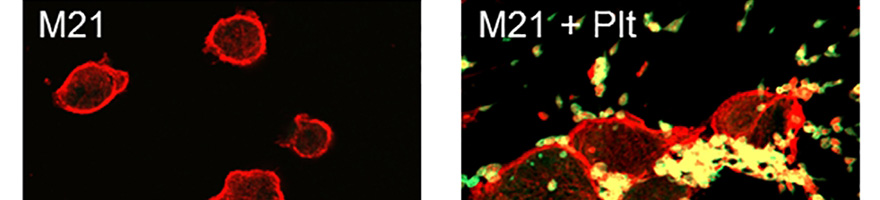
In the present study, the research team dissected the role of platelet αIIb integrin (GPIIb) for early and late steps in pulmonary melanoma metastasis. They first addressed in vitro potential mechanisms for initial recruitment of circulating melanoma cells to vascular endothelium using a flow chamber model and then assessed the role of GPIIb for metastasis formation in vivo in mice lacking integrin αIIb (GPIIb-/-). GPIIb associates with GPIIIa (integrin β3) to form the platelet-specific integrin heterodimer GPIIb-IIIa (integrin αIIbβ3), representing the most abundant platelet surface receptor and predominantly functioning as platelet fibrinogen receptor. By binding to fibrinogen, but also to von Willebrand factor, GPIIb-IIIa mediates cross-linking of adjacent platelets, resulting in platelet aggregation and platelet secretion of chemokines as well as growth factors. Moreover, binding of GPIIb-IIIa to fibronectin, vitronectin or PECAM-1 leads to platelet adhesion to the vessel wall. In order to follow the initial steps of tumor metastasis in wildtype (WT) and GPIIb-deficient mice, they applied a novel microscopic approach using a fluorescence optical imaging system based on laser scanning confocal technology.
They showed that the acute retention of malignant melanoma cells is dramatically reduced in mice deficient in platelet GPIIb and also they found that GPIIb has a minor effect of adhesion of single melanoma cells, but rather mediates the formation of platelet-rich melanoma cell aggregates, which are retained in the pulmonary vasculature.
Read more